

Synonyms: inosine 5'-monophosphate dehydrogenase, type 2, IMP dehydrogenase, type II, IMPDH2.
Inosine 5'-monophosphate dehydrogenase type 2 (IMPDH 2, E.C.1.1.1.205) is the predominant isoform of IMPDH and a validated target to treat a wide range of cancers and infectious diseases and to prevent lymphocytes proliferation.
Catalytic activity
Inosine Monophosphate Dehydrogenase (IMPDH) converts inosine 5'-monophosphate (IMP) to
xanthosine 5µ-monophosphate (XMP) using NAD+ as a cofactor.
The oxidation of IMP to XMP is considered as the pivotal step in the biosynthesis of guanine nucleotide, whose pool controls cell proliferation and many other major cellular processes(1). The decrease in guanine nucleotide resulting from IMPDH inhibition interrupts the nucleic acid synthesis in proliferating cells. The involvement of IMPDH in de novo guanine nucleotide biosynthesis makes IMPDH a crucial enzyme in cell proliferation and differentiation(2). IMPDH is recognized as a validated target for several major therapeutic areas. IMPDH inhibitors are exploited as antiviral (e.g. ribavirine), antiparasitic, antimicrobial, antileukemic, and immunosuppressive agents(2). IMPDH Type II is the predominant isoform of the enzyme and is selectively expressed in proliferating cells, including lymphocytes and tumor cells(2).
NOVOCIB's IMPDH 2 has been cloned by RT-PCR amplification of mRNA extracted from human hepatoma cells (NP_000875.2, 100% identity) and expressed in E.coli.
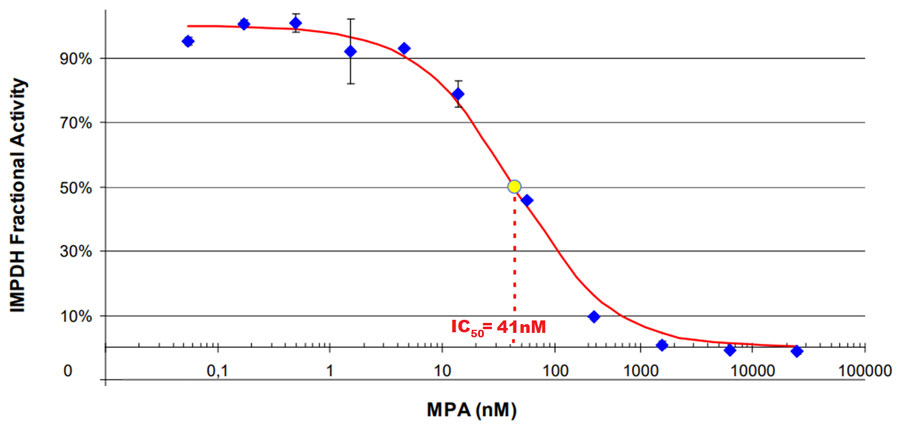
NOVOCIB's purified IMPDH 2 is an active enzyme characterized for its affinity for inosine 5'-monophosphate and NAD substrates, and its sensitivity to enzyme inhibitors such as mycophenolic acid and ribavirine-monophosphate.
Unit Definition: One unit of IMPDH Type II catalyzes the oxydation of 1 µmole of IMP to XMP per minute at pH 8.8 at 37 µC.
High Specific Activity: > 200 mU/mg protein.
| #REF | SIZE | PRICE | |
|---|---|---|---|
| #E-Nov1-100 | Human IMPDH Type 2 100mU |
295.00 € |
295.00 €
Inquiry
|
| #E-Nov1-250 | Human IMPDH Type 2 250mU |
550.00 € |
550.00 €
Inquiry
|
| #Screening E-Nov1 | Human Recombinant IMPDH2 Screening Service IC50 determination for new compounds, performed in duplicate, with mycophenolic acid as the positive control. |
590.00 € |
590.00 €
Inquiry
|
Last updated on : December 8th, 2025.
You can ask us for a quotation here or write at contact@novocib.com
Download our brochure "NovoCIB's IMPDH Products & Services"
Assay condition: KH2PO4 0.1M, pH8.8, NAD 250µM, DTT 2.5mM, 2.5mU/ml of human recombinant IMPDH II, Incubation at 37µC. Reaction started by adding IMP at 250µM final concentration. NADH formation was followed in an iEMS Reader MF (Labsystems) plate reader at 340nm.

Download this Document: "NOVOCIB's Human Recombinant IMPDH"
Download this Document: "NOVOCIB - IMPDH Products & Services"
*For best results, always use freshly prepared solutions of DTT, IMP, and NAD.
| Time (min) | IMP 1 mM, no NAD | IMP 1 mM, no NAD (duplicate) | IMP 1 mM + 1 mM NAD | IMP 1 mM + 1 mM NAD (duplicate) |
|---|---|---|---|---|
| 0 | 0.118 | 0.116 | 0.167 | 0.165 |
| 1 | 0.118 | 0.116 | 0.205 | 0.203 |
| 2 | 0.119 | 0.117 | 0.244 | 0.240 |
| 3 | 0.120 | 0.116 | 0.283 | 0.278 |
| 4 | 0.121 | 0.116 | 0.322 | 0.316 |
| 5 | 0.121 | 0.116 | 0.362 | 0.354 |
| 6 | 0.121 | 0.116 | 0.401 | 0.392 |
| 7 | 0.122 | 0.116 | 0.440 | 0.430 |
AU/min: 0.0382
To validate IMPDH2 inhibitors identified in vitro, try our analytical service that measures guanine nucleotide depletion and related metabolic changes. These assays confirm whether compounds active in biochemical screens also suppress nucleotide synthesis in living cells.
IMPDH plays a critical role in lymphocyte proliferation and immune response modulation, making it a prime target for:
IMPDH Type 2 is overexpressed in many cancer cells, making it an attractive target for anticancer therapies:
The continued elucidation of IMPDH's atomic structure and mechanism of action continues to drive the development of more potent and selective inhibitors, opening new possibilities for targeted therapies across multiple disease areas.









Find answers to common questions about our analytical services
Have more questions? Our team is here to help.
Contact Us